Abstract
Background: MM and AL are the two most common malignant monoclonal gammopathies. Both diseases result from the accumulation of clonal PCs, but their clinical behavior is significantly different suggesting fundamental differences in disease biology. Previous attempts to identify genetic hallmarks that could explain such differences have been unsuccessful. Furthermore, it is unknown if MM and AL arise from the same or different normal PC counterparts.
Aim: To define a transcriptional atlas of the normal PC development in peripheral blood (PB) and bone marrow (BM) for comparison with the transcriptional programs of clonal PCs in MM and AL.
Methods: A total of 93 subjects were studied. In 7 healthy adults (HA), PB PCs were phenotypically sorted according to heavy-chain isotypes (IgG, IgA and IgM). In addition, 5 different BM PCs subsets were isolated based on the differential expression of CD19, CD39, CD81 and CD56, due to their ascribed role in dissecting unique BM PC differentiation states. Clonal PCs from patients with MM (n=38) and AL (n=41) were isolated by FACS according to patient-specific aberrant phenotypes. Due to small numbers of PCs sorted from each subset in HA and clonal PCs in AL patients, we used an RNAseq method optimized for limited cell numbers. Differential expression across all pairwise comparisons between groups was analyzed with Deseq2 R package followed by k-means clustering of genes in R. Single-cell RNAseq (scRNAseq, 10xGenomics) was performed in a total of 35,910 PCs from 3 HA, 2 MM and 2 AL. We used Seurat R package to remove batch effect followed by canonical correlation to perform an integrated analysis of all single PCs from HA, MM and AL subjects.
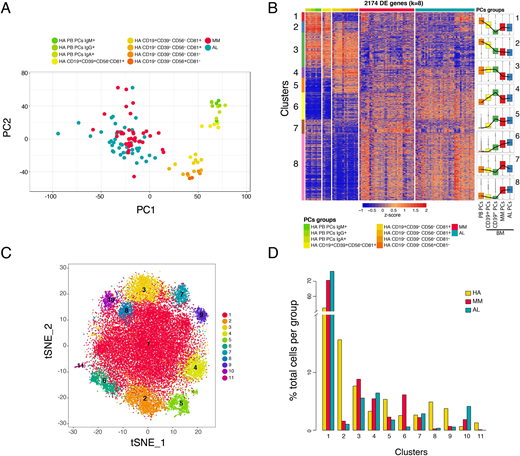
Results: Principal component analysis of RNAseq data unveiled two major clusters of normal PCs: those in PB and those in BM (with some transcriptional diversity between CD19+ and CD19- PCs), whereas the CD19+CD39+CD81+CD56- BM subset co-localized with PB and CD39- BM PCs (Panel A). Clonal PCs from MM and AL patients clustered together, and both displayed some transcriptional variance related to the spatial location of normal PCs (i.e. PB or BM). In total, 2174 genes were found significantly deregulated after cross-comparing the 10 PC groups (adj.p-value<0.01, logFC>1) and semi-supervised k-means clustering unveiled 8 transcriptional modules (Panel B). Namely, the transition from PB into BM PCs was characterized by genes related to proliferation (clusters 1 & 2), whereas CD39+ and CD39- BM PC subsets differed on the expression of genes associated with proliferation, homing, and metabolism (1, 2, 4 & 6). Thus, CD19+CD39+CD81+CD56- BM PCs emerged as a novel subset that bridges new-born PB with long-lived (CD39-) BM PCs. Interestingly, clonal PCs from MM and AL shared transcriptional programs related to quiescence (5 & 6) with long-lived BM PCs; however, skewing of polyclonal immunoglobulin gene expression (3) and active gene transcription (8) emerged as hallmarks of the neoplastic transformation from normal, long-lived PCs into clonal PCs. That notwithstanding, the later displayed expression levels of the proliferation and homing transcriptional modules (1 & 4) similar to new-born PB and CD39+ BM PCs. Of note, a small transcriptional cluster of genes related to ribosome biogenesis (7) was significantly more expressed in MM than AL. These findings led us to integrate scRNAseq profiles of normal and clonal BM PCs from MM and AL patients, to define PC clusters based on their transcriptional program rather than their normal vs malignant status (Panel C). This strategy unveiled 11 different PC clusters with unequal distribution between groups. Thus, more than half of clonal PCs in MM and AL were assigned to a cluster that is also predominant in normal PCs (1). By contrast, other clusters with a transcriptional program similar to that of new-born PCs (2 & 5) became rarer in MM and AL. Furthermore, a cluster of PCs with an immature-like phenotype (6) was detectable in MM but almost absent in AL.
Conclusions: This is the first integrated analysis of the transcriptional programs of normal PC subsets and clonal PCs in MM and AL, both at the bulk and single-cell levels. Our results unveil shared and exclusive transcriptional states in normal and clonal PCs, together with unique differences between clonal PCs in MM and AL. Thus, we provide here a fundamental resource to understand normal PC development and the cellular origin of both malignant monoclonal gammopathies.
Puig:Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Ocio:Pharmamar: Consultancy; AbbVie: Consultancy; Janssen: Consultancy, Honoraria; Seattle Genetics: Consultancy; BMS: Consultancy; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Mundipharma: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Array Pharmaceuticals: Research Funding. Oriol:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Martinez Lopez:Bristol Myers Squibb: Research Funding, Speakers Bureau; Janssen: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Celgene: Research Funding, Speakers Bureau. Mateos:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lahuerta:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. San-Miguel:Sanofi: Consultancy; Takeda: Consultancy; Novartis: Consultancy; MSD: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Brystol-Myers Squibb: Consultancy; Amgen: Consultancy; Roche: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal